Can Research Subject to Subpart B and That Includes
B Assent means a childs affirmative agreement to. 46204 d For research that holds out the prospect of direct benefit to the pregnant woman the prospect of direct benefit both to the pregnant woman and fetus or no prospect of benefit for the woman nor the fetus when risk to the fetus is not greater than minimal and the purpose of the research is provide important.
Each of the exemptions at this section may be applied to research subject to subpart B if the conditions of the exemption are met.
. Regarding the reasonably foreseeable impact of the research on the fetus or neonate. INSTITUTIONAL REVIEW BOARD Investigator Guidance Series THE UNIVERSITY OF UTAH Please contact the IRB Office at 801 581-3655 or irbhscutahedu for additional guidance. Note that the law for non-exempt human subjects as research subject to 45 CFR 46 Subpart B are the ones that involves the use of pregnant women human fetuses or neonates.
Some other basic requirements of Subpart A as shown in C is that Research that pertains to. Each of the exemptions at this section may be applied to research subject to subpart B if the conditions of the exemption are met. 21 CFR Subpart B - Protection of Researchers and Research Subjects.
223 Patient access and restrictions on use. 23 CFR 420107 SPR Subpart B. 1 To provide assistance to State educational agencies and local educational agencies in establishing reading programs for students in kindergarten through grade 3 that are based on scientifically based reading research to ensure that every student can read at grade level or above not later.
Not less than 25 percent of the funds set aside by 23 USC. 221 Relationship to Federal statutes protecting research subjects against compulsory disclosure of their identity. 46206 Research involving after delivery the placenta the dead fetus or fetal material.
Considering the situation and based on the laws end regulation codes established by the United States Department of Health and Human Services IRB has the authority to approve research involving pregnant women. Subpart 1reading first 6361. Research conducted in established or commonly accepted educational settings involving normal educational practices such as a research on regular and special education instructional strategies or b research on the effectiveness of or the comparison among instructional techniques curricula or classroom management methods.
The Federal cost share is 80 percent unless the Secretary determines that the interests of the Federal-aid. 46207 Research not otherwise approvable which presents an opportunity to understand prevent or alleviate a serious problem affecting the health or welfare of pregnant women fetuses or neonates. For research subject to the 2018 Common Rule research involving prisoners cannot be deemed exempt under 45 CFR 46104d except for research aimed at involving a broader subject population that only incidentally includes prisoners.
1 Subpart B. The Common Rule includes additional protections for certain vulnerable research subjects. The exemptions at this section do not apply to research subject to subpart C except for research aimed at involving a broader subject population that only incidentally includes prisoners.
46402a who are pregnant assent and permission are obtained in accord with the provisions of the Protections for Children Involved as Subjects Subpart D. 46205 Research involving neonates. This includes most research on regular and special education instructional strategies and research on the effectiveness of or the comparison among instructional techniques curricula or.
A Children are persons who have not attained the legal age for consent to treatments or procedures involved in the research under the applicable law of the jurisdiction in which the research will be conducted. Important Notes about Category 3 Deception is allowed if certain criteria are met. The exemptions at this section do not apply to research subject to subpart C except for research aimed at involving a broader subject population that only incidentally includes prisoners.
Each of the exemptions may be applied to research subject to subpart B if the conditions of the exemption are met. Subpart B provides additional protections for pregnant women in vitro fertilization and fetuses Subpart C contains additional protections for prisoners Subpart D does the same for children. Can research subject to Subpart B and includes pregnant women as subjects exempt.
131623 Confidentiality of identity of research subjects. Research involving the use of. IRB Approval under Subpart B.
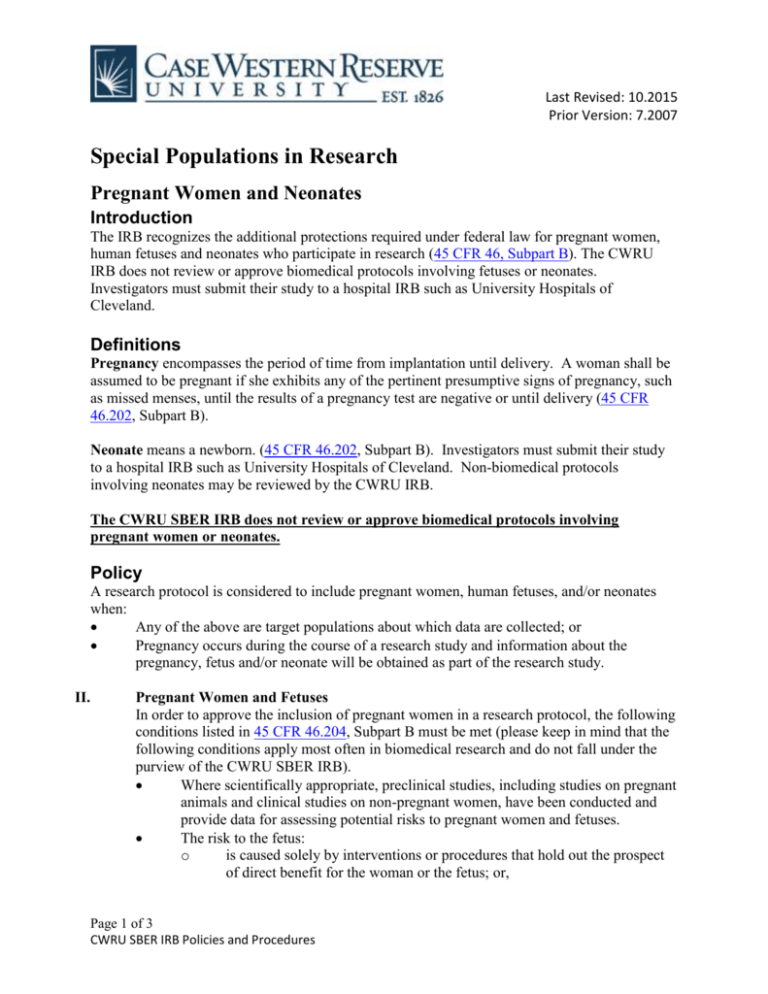
For DHHS-funded research 45 CFR subpart B applies to all research involving pregnant women. The definitions in 46102 of subpart A shall be applicable to this subpart as well. 222 Notice to patients of Federal confidentiality requirements.
When conducting research subject to 45 CFR 46 Subpart B eg involving pregnant women human fetuses and neonates Principal Investigators PIsLead Site Investigators must ensure that the protocol and the performance of the research is in compliance with all applicable sections of Subpart B and the other applicable Subparts of 45 CFR 46 as described in Section. For children as defined in Sec. 505a each fiscal year shall be expended by the State for research development and technology transfer activities.
801 who intends to maintain the confidentiality of the. DHHS Regulations are provided in 45 CFR Part 46. Yes each of the exemptions in 45 CFR 46104 may be applied to research subject to Subpart B.
The purposes of this subpart are as follows. In addition as used in this subpart. Prev next 131621 Definitions.
Subpart B Pregnant women fetuses and neonates. 131624 Exemption from prosecution for researchers. IRB an acronym for Institutional review board under Subpart B Section 46203 stated that IRB has the jurisdiction to determine whether to approve.
13 October 2021 by seth mipworth Can research subject to subpart b and that includes pregnant women as subjects be exempt from the regulations per 45 can research subject to subpart b and that includes pregnant women as subjects be exempt from the regulations per 45 cfr 46 if all of the conditions of the exemption are met. A research study will compare a new combined behavioral and pharmacologic treatment with the standard behavioral treatment alone for individuals. When the research is subject to Subpart D and includes children Category 2 still does not allow surveys or interviews or the observer participating with children public behavior observation without intervention is permitted.
According to 45 CFR subpart B pregnant women or fetuses may be involved in research funded by DHHS if all of the following conditions are met. Each of the exemptions at this section may be applied to research subject to subpart B if the conditions of the exemption are met. Where scientifically appropriate pre-clinical studies including studies on.
Informed consent can be waived or altered in research involving prisoners only in. A Any person conducting a bona fide research project directly related to the enforcement of the laws under the jurisdiction of the Attorney General concerning drugs or other substances which are or may be subject to control under the Controlled Substances Act 84 Stat.

1 Fcc Part 15 Subpart B Class B Radiated Emissions Limits At 3 M Download Table
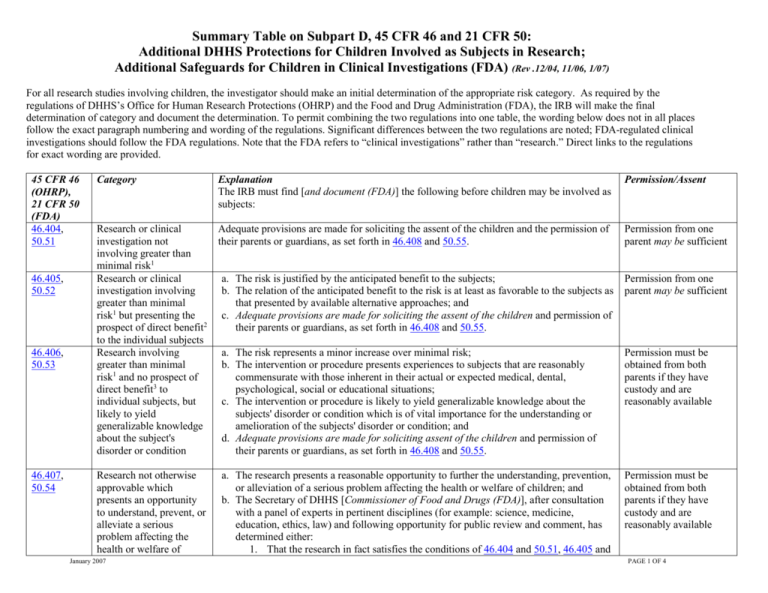
Summary Table On 45 Cfr 46 Subpart D
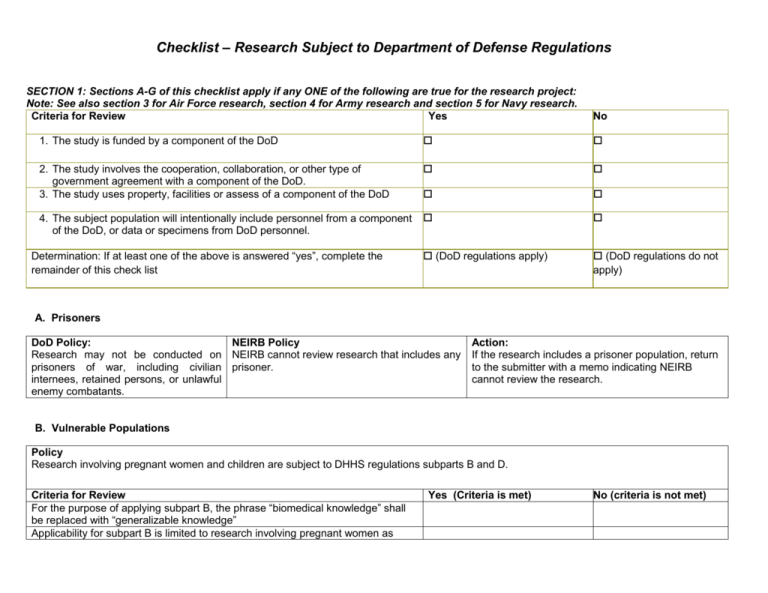
Checklist Research Subject To Department Of Defense Regulations

Worksheet For Studies Involving Pregnant Women Fetuses And Neonates

November 2008 What Is A Vulnerable Subject

1 Fcc Part 15 Subpart B Class B Radiated Emissions Limits At 3 M Download Table

Lausd Report Card Template Student Report Card A Self Reflection By Eric Jayne Report Card Report Card Template Upper Elementary Math
Top Left Dc Capacitor Bank With Duplicated Dc Bus Connections One Download Scientific Diagram
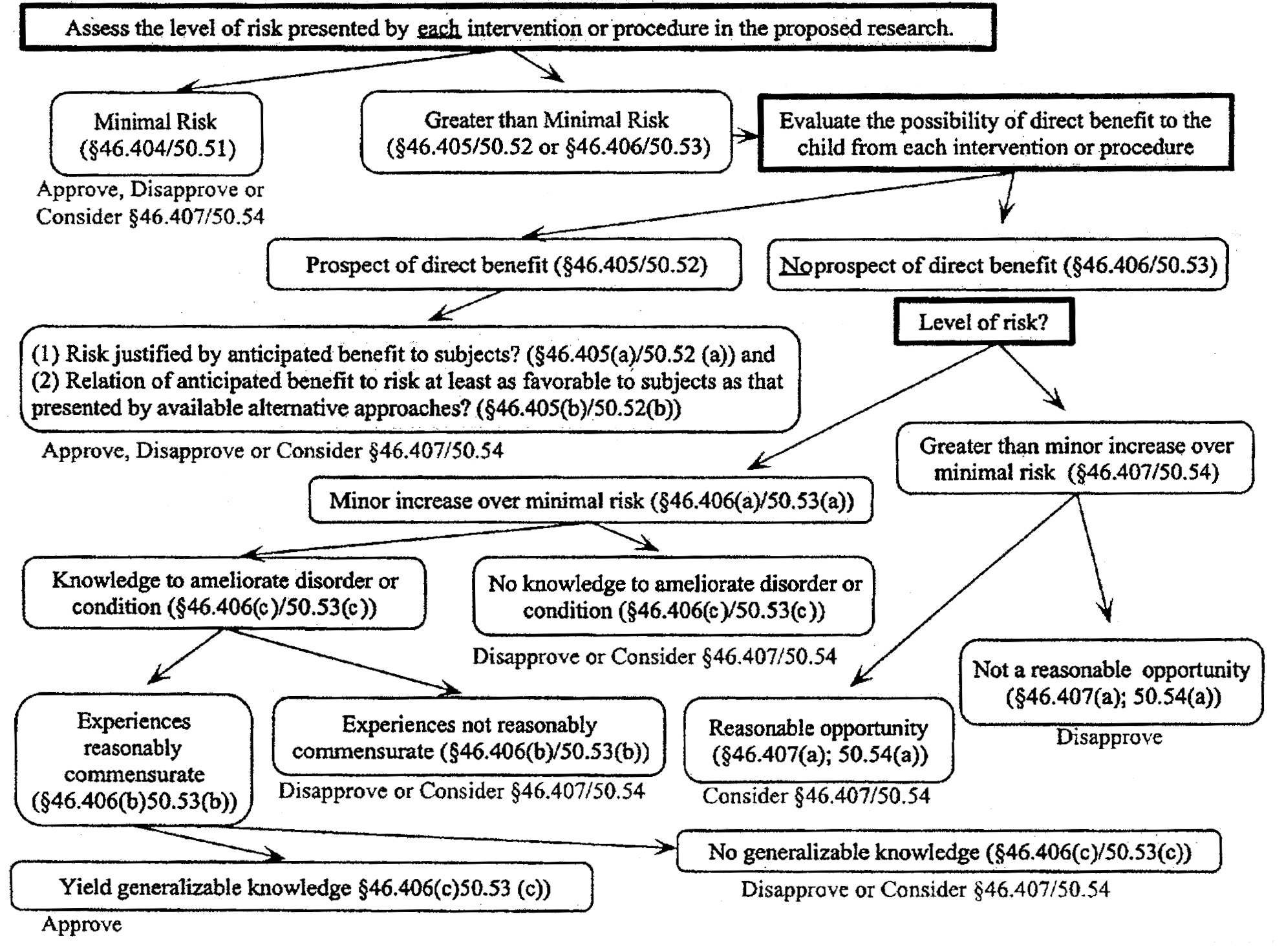
Appendix A Algorithm For Subpart D Analysis 45 Cfr 46 And 21 Cfr 50 Hhs Gov

Solved Part 2 Research Subpart 2 Choose Either A Or B And Chegg Com

Soil Classification Wikipedia The Free Encyclopedia Soil Classification Soil Texture Triangle Worksheet


Comments
Post a Comment